- Home
- CASOS DE ÉXITO
- Radiocirugía giroscópica para el tratamiento de la neuralgia del trigémino
Resumen
Introducción: El tratamiento radiocirúrgico de la neuralgia del trigémino (NT) es el procedimiento de radiocirugía funcional más común utilizado para modular el dolor refractario. El objetivo de este estudio es describir los resultados clínicos y los parámetros dosimétricos de la primera serie de pacientes con NT tratados con radiocirugía giroscópica sin marco ZAP-X (ZAP Surgical Systems, Inc., San Carlos, CA), un innovador dispositivo de radiocirugía sin marco.
Métodos: Un total de 30 pacientes con NT recibieron radiocirugía giroscópica sin marco (GRS) entre febrero de 2023 y enero de 2025. Se desarrollaron planes de tratamiento que administraban una dosis máxima de 90 Gy en un objetivo que cubría un segmento de 5 mm del nervio trigémino en la localización retrogasseriana utilizando un único isocentro (colimador de 5 mm). Se analizó la información clínica y del tratamiento, prestando especial atención a las características demográficas, la etiología, los tratamientos previos, la escala de intensidad del dolor del Instituto Barrow (BNI) antes y después de la GRS (respuesta al alivio del dolor), el tiempo hasta el alivio del dolor y la tasa de complicaciones y recidivas.
Resultados: 30 pacientes (21 mujeres y 9 hombres) con una mediana de edad de 66 años (rango: 31-92 años) se sometieron a GRS por NT refractaria. La mediana del tiempo transcurrido desde el primer diagnóstico hasta la GRS fue de 4 años (rango: 6 meses a 18 años). Todos los pacientes habían recibido una primera línea terapéutica farmacológica, 15 pacientes recibieron al menos una termocoagulación por radiofrecuencia, 7 pacientes recibieron bloqueos nerviosos, 4 pacientes fueron tratados con inyecciones de bótox y 2 pacientes habían recibido radiocirugía previa. La mediana de la escala de intensidad del dolor BNI antes de la GRS fue IV (rango IIIa-V). La mediana del tiempo de tratamiento fue de 37 minutos, con una mediana de 221 haces. La mediana de V12 y V10 del tronco encefálico fue de 0,04 cc (rango: 0-0,16 cc) y 0,07 cc (rango: 0-0,24 cc), respectivamente. La mediana de la escala de intensidad del dolor BNI después de la GRS fue II (rango: I-VI), y el 80 % de los pacientes no refirieron dolor o solo dolor ocasional sin medicación. La mediana del tiempo hasta el alivio del dolor fue de 15 días (rango: 1-60 días). Cinco pacientes (16,6 %) presentaron hipoestesia facial ipsilateral después de la GRS. El 10 % de los pacientes presentaron dolor recurrente entre 2 y 6 meses después de la GRS.
Conclusiones: La GRS es una herramienta precisa para el tratamiento de la NT, que ha demostrado su seguridad y eficacia en lo que, según nuestro conocimiento, es la primera serie de casos documentada que describe su uso.
Introducción
La neuralgia del trigémino (NT) es un dolor facial intenso que se diagnostica en un entorno clínico. Los síntomas se describen como un dolor unilateral, similar a una descarga eléctrica, punzante, de aparición repentina y remisión espontánea, sin déficit neurológico objetivo. La NT puede estar causada por un tumor, esclerosis múltiple o anomalías estructurales de la base del cráneo, como compresión vascular. La NT idiopática se refiere a todas las causas sin una etiología establecida. La primera línea terapéutica es farmacológica. No obstante, la NT refractaria puede convertirse en un reto multidisciplinar [1,2].
Cuando se administra radiación de forma focalizada al tejido cerebral en dosis subablativas, la actividad neural puede verse alterada. Cuando se realiza en un nodo o conexión específica del circuito cerebral, se denomina radiomodulación. Régis et al. propusieron que la radiocirugía estereotáctica (SRS) podría producir un efecto de modulación no ablativa en diferentes patologías, mostrando de forma consistente un efecto clínicamente positivo en el tratamiento del dolor, la epilepsia o los síntomas de temblor inmediatamente después del tratamiento o poco después, sin generar una lesión permanente en los circuitos neuronales [2-4].
La radiocirugía estereotáctica (SRS) del nervio trigémino se utiliza ampliamente para tratar el dolor facial refractario. Este tratamiento se caracteriza por la administración de dosis altas (60 a 97 Gy) en un objetivo específico, normalmente a lo largo del recorrido del nervio en la cisterna prepontina. Una revisión sistemática de la literatura centrada en los resultados de la SRS para la NT encontró 65 estudios que informaban sobre 6461 pacientes con una respuesta mediana de ausencia de dolor (FFP) con o sin medicación del 79 % o superior. Aunque la mayoría de los casos descritos se trataron con SRS con marco, la SRS con máscara y guiada por imágenes mediante aceleradores lineales (LINAC) también ha demostrado ser una opción de tratamiento segura, eficaz y menos invasiva para la NT. La escala de intensidad del dolor del Barrow Neurological Institute (BNI) (I: sin dolor; II: dolor ocasional, que no requiere medicación; III: algo de dolor, controlado con medicación; IV: algo de dolor, no controlado con medicación; V: dolor intenso/sin alivio del dolor) es la clasificación más utilizada para informar sobre los resultados tras la SRS [1,5].
ZAP-X® (ZAP Surgical Systems, Inc., San Carlos, California) es un nuevo sistema de radiocirugía basado en LINAC y guiado por imágenes, dedicado al tratamiento de lesiones intracraneales y de la columna cervical. Este sistema cuenta con un LINAC de banda S de 3,0 megavoltios (MV) montado dentro de una combinación de cardanes acoplados que giran con precisión alrededor de un isocentro común, realizando una irradiación giroscópica. Para dar forma al haz se utilizan colimadores cónicos de entre 4 y 25 mm de diámetro. Es «autoprotegido», ya que casi toda la radiación queda contenida dentro del dispositivo, lo que permite su uso seguro sin necesidad de una cámara de radioterapia [3,6,7].
El objetivo de este estudio es describir los resultados clínicos de la primera serie de pacientes con TN refractario tratados con radiocirugía giroscópica sin marco (GRS) ZAP-X, una tecnología innovadora dedicada a la SRS, diseñada específicamente para administrar con precisión dosis altas a objetivos intracraneales milimétricos.
Materiales y métodos
Entre febrero de 2023 y enero de 2025, se sometió a GRS a una serie de 30 pacientes con NT. Se consideraron candidatos todos los pacientes con NT refractaria, independientemente de la etiología. Se recopiló y analizó en una base de datos la información sobre la edad, el sexo, la fecha del diagnóstico, la etiología, los tratamientos previos, la escala de intensidad del dolor de BNI antes y después de la GRS (respuesta al alivio del dolor), el tiempo hasta el alivio del dolor y la tasa de complicaciones y recidivas.
Se administró una dosis máxima de 90 Gy (prescrita a la línea de isodosis del 100 %) dentro de un objetivo que cubría un segmento de 5 mm del nervio trigémino en la localización retrogasseriana utilizando un único isocentro. Se consideró obligatorio un V70 ≥ 50 % para el volumen objetivo. Aunque ZAP-X tiene un colimador de 4 mm, se seleccionó el colimador de 5 mm para el tratamiento de esta primera serie con el fin de garantizar que se cubriera todo el objetivo, el grosor del nervio y las incertidumbres del movimiento del nervio. Se contornearon el cerebro sano, la vía óptica, el tronco encefálico, el lóbulo temporal ipsilateral y las cócleas como órganos en riesgo (OAR), y se aplicaron restricciones de dosis siguiendo las directrices internacionales. Para el tronco encefálico, se dio prioridad a V12 <0,2 cc y V10<0,5 cc con el fin de evitar la necrosis del tronco encefálico [8,9].
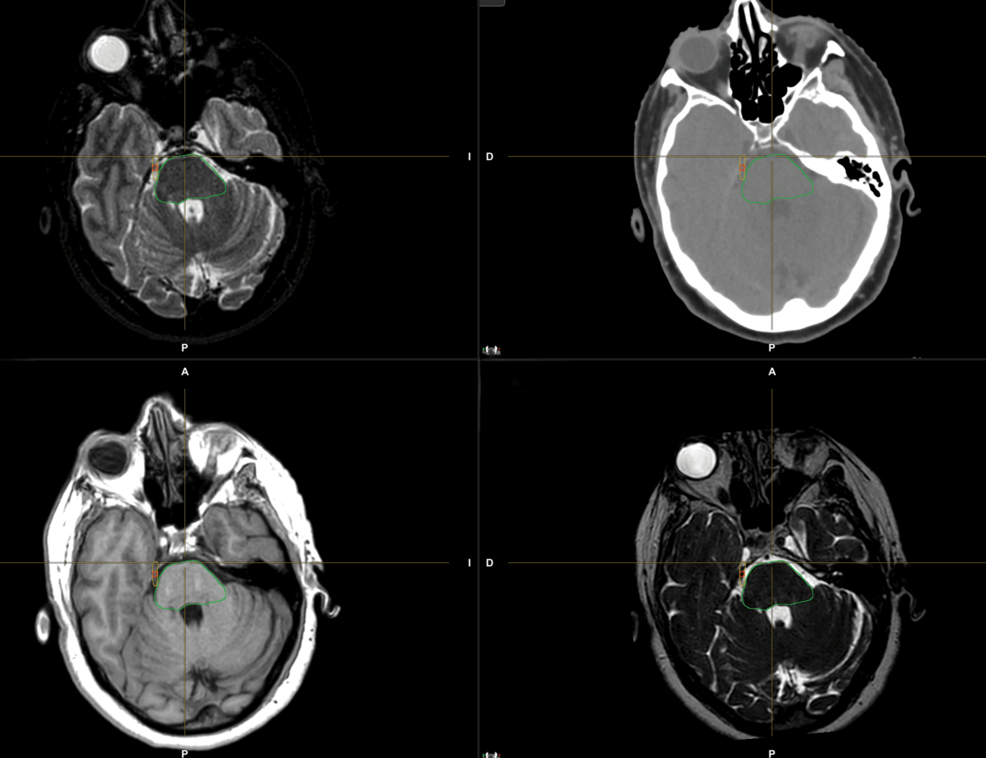
En la figura 1 se muestra un ejemplo de delimitación del volumen objetivo.
Figura 1: Delineación del volumen objetivo en el nervio trigémino derecho.
Para la delineación del volumen, se adquirieron secuencias 3D T1, T2 y FIESTA de tomografía computarizada (TC) y resonancia magnética (RM) de 1,5 teslas sin contraste en posición supina. Se seleccionó un grosor de corte inferior o igual a 1 mm para ambas imágenes, a fin de garantizar una resolución espacial suficiente. Se utilizó una máscara termoplástica para la inmovilización. El registro rígido y deformable, junto con el contorneado, se realizó en el software Brainlab Elements (Brainlab AG, Múnich, Alemania), utilizando todas las secuencias para identificar correctamente toda la extensión del nervio trigémino y determinar mejor el volumen objetivo.
Para la planificación del tratamiento, la plataforma ZAP-X GRS funciona con un sistema específico denominado ZAP-X TPS, que está integrado en el sistema de administración ZAP-X. El cálculo de la dosis se basó en el algoritmo de trazado de rayos. Se utilizó una combinación de técnicas de planificación directa para colocar manualmente los disparos y una optimización de la planificación inversa para obtener una solución óptima en cuanto a la disposición de los haces. Los planes de tratamiento fueron revisados por un oncólogo radioterápico, un neurocirujano y un físico médico, que evaluaron la distribución de la isodosis y revisaron el histograma dosis-volumen (DVH) del volumen objetivo y los OAR [10].
La localización del objetivo se consigue mediante un sistema integrado de imágenes planas de kilovoltios (kV) que proporciona un registro tridimensional del paciente. Se realiza una realineación automática antes y durante el tratamiento, lo que permite una configuración y un seguimiento sin marco. La tasa de dosis es de 1500 MU por minuto. Además, la utilización de una energía muy baja y una distancia fuente-eje (SAD) muy corta, de 45 cm, optimiza una rápida caída de la dosis periférica y un gradiente de dosis pronunciado. Además, una cámara de ionización de monitorización de dosis mide la cantidad de radiación administrada en tiempo real para garantizar la precisión del tratamiento [3,6,7].
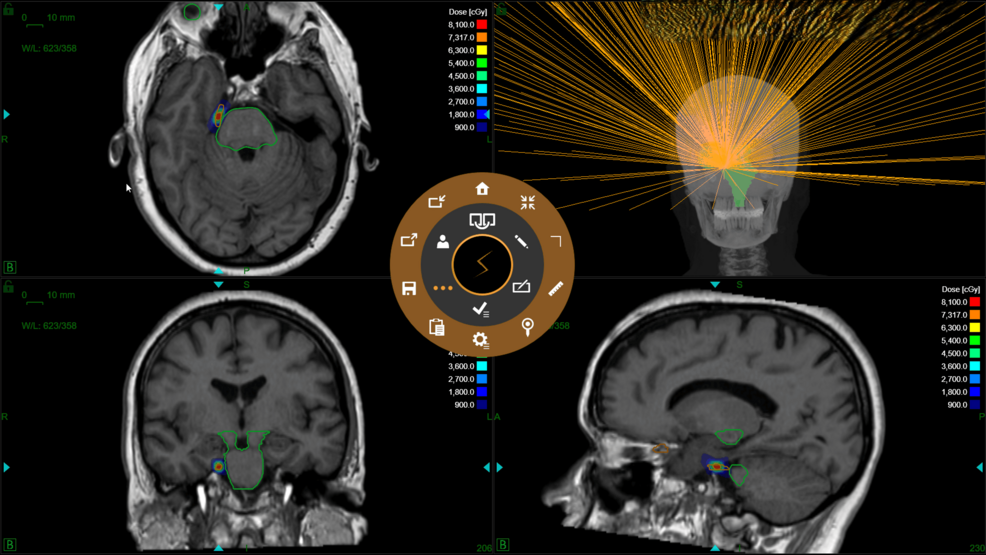
En la figura 2 se muestra un ejemplo de distribución de isodosis.
Figura 2: Distribución de la dosis y evaluación del haz en un paciente con neuralgia del trigémino derecho.
El primer seguimiento clínico se programó dos semanas después del tratamiento y, en función de la respuesta al alivio del dolor, se aplicó un protocolo personalizado de reducción de la medicación. En cada visita se evaluó la escala de intensidad del dolor de la BNI y las toxicidades relevantes. Se preguntó a los pacientes sobre síntomas como hipoestesia, sequedad ocular, anestesia corneal o disestesia.
Se realizó un análisis descriptivo de los datos recopilados, creando tablas de frecuencia sobre las variables cualitativas y describiendo la mediana y el rango de las variables cuantitativas.
Resultados
Un total de 30 pacientes (21 mujeres y 9 hombres) con una mediana de edad de 66 años (rango: 31-92 años) se sometieron a GRS por NT refractaria. En cuanto a la etiología, la mitad de la serie (15 pacientes) se clasificó como idiopática, sin describir ninguna causa conocida relacionada con el inicio del dolor. Por otro lado, 6 pacientes desarrollaron NT tras una intervención dental (extracción o manipulación), 4 pacientes presentaban NT relacionada con tumores benignos con compresión nerviosa (meningiomas), 3 pacientes tenían esclerosis múltiple, un paciente desarrolló NT tras una meloplastia y un paciente tras un herpes zóster trigémino.
La mediana del tiempo transcurrido desde el primer diagnóstico hasta la GRS fue de 4 años (rango: 6 meses a 18 años). Todos los pacientes habían recibido una primera línea terapéutica farmacológica, 15 pacientes recibieron al menos una termocoagulación por radiofrecuencia, siete pacientes recibieron bloqueos nerviosos, cuatro pacientes fueron tratados con inyecciones de bótox y dos pacientes habían recibido radiocirugía previa. La mediana de la escala de intensidad del dolor BNI antes de la GRS fue IV (rango: IIIa-V).
En cuanto a las características del tratamiento, la mediana del tiempo de tratamiento fue de 37 minutos (rango: 31-45 minutos) utilizando una mediana de 221 haces (rango: 98-395 haces). La gran variabilidad en cuanto al tiempo de tratamiento y el número de haces dependía del número de trayectorias utilizadas para la disposición de los haces durante la planificación del tratamiento (6-17 trayectorias). Dado que la anatomía del nervio y la relación con el tronco encefálico variaban entre los pacientes, cuanto menor era la distancia del objetivo con el tronco encefálico, mayor era el número de trayectorias y haces necesarios para configurar de forma óptima la distribución de la dosis, optimizando la cobertura del objetivo y el gradiente de dosis alrededor del tronco encefálico. La mediana de V12 y V10 del tronco encefálico fue de 0,04 cc (rango: 0-0,16 cc) y 0,07 cc (rango: 0-0,24 cc), respectivamente. La mediana de V70 del volumen objetivo fue del 87 % (rango: 62-100 %). La mediana de D0,03 cc en el lóbulo temporal ipsilateral fue de 13 Gy (rango: 5-25 Gy). La mediana de la dosis media en el nervio trigémino fue de 39 Gy (rango: 34-52 Gy).
Con una mediana de seguimiento de ocho meses (rango: 2-24 meses), el 100 % de los pacientes mostraron al menos alguna mejoría tras la GRS. La mediana de la escala de intensidad del dolor BNI tras la GRS fue II (rango: I-VI), y el 80 % de los pacientes no refirieron dolor o solo dolor ocasional sin medicación. La mediana del tiempo hasta el alivio del dolor fue de 15 días (rango: 1-60 días). Se produjo recurrencia del dolor en 3 pacientes (10 %) entre 2 y 6 meses después de la primera GRS. Dos de ellos eran candidatos aptos para una segunda GRS, ya que había un intervalo de seis meses entre el primer tratamiento, y ambos pacientes mostraron al menos una escala de mejora después de la segunda GRS.
Las complicaciones tras el tratamiento fueron leves; cinco pacientes (16,6 %) presentaron hipoestesia facial ipsilateral tras la GRS (de ellos, dos habían recibido previamente una SRS y un paciente ya presentaba una hipoestesia leve antes del tratamiento).
Discusión
La neuralgia del trigémino (NT) sigue siendo una de las afecciones dolorosas más difíciles de tratar, especialmente en los casos refractarios. El estudio que aquí se presenta examina la primera serie de pacientes tratados con ZAP-X® GRS.
La cohorte de pacientes incluía una variedad de etiologías de NT. Esta amplia representación de subtipos de NT refuerza la generalización de los resultados, lo que indica que la GRS puede ser beneficiosa para pacientes con diversas causas subyacentes de NT. La mayoría de los pacientes de esta serie habían recibido tratamientos farmacológicos previos o terapias invasivas, como termocoagulación por radiofrecuencia o bloqueos nerviosos, lo que pone de relieve la naturaleza refractaria de su afección. La mediana de la escala de intensidad del dolor BNI antes del tratamiento fue IV, lo que refleja la gravedad del dolor que experimentaban estos pacientes antes de la GRS, y la mayoría de los pacientes informaron de una mejora significativa en los niveles de dolor tras el tratamiento.
La mediana de la escala de intensidad del dolor BNI tras la GRS mejoró a II, lo que demuestra que una parte sustancial de los pacientes experimentó un alivio significativo. Aunque es necesario un seguimiento más prolongado para validar estos resultados, la alta tasa y el tiempo medio hasta el alivio del dolor son coherentes con los informes anteriores sobre la SRS para la NT. El alivio casi inmediato del dolor en un subgrupo de pacientes no puede relacionarse con cambios celulares que requieren varios meses para manifestarse. Esta observación sugiere que la SRS puede modular la transmisión neuronal en neuronas aparentemente intactas [1,4,10-14].
La precisión en la administración de la radiación es una característica distintiva del sistema ZAP-X. El proceso de planificación del tratamiento con ZAP-X requirió una atención especial a la distribución de la dosis. Esto es especialmente importante en el contexto de la NT, donde el objetivo es administrar una radiación suficiente para modular el nervio trigémino, evitando dañar estructuras críticas como el tronco encefálico. En esta primera serie se eligió el colimador de 5 mm en lugar del de 4 mm para garantizar una cobertura adecuada y, a pesar del mayor tamaño del colimador, los valores medios de V12 y V10 para el tronco encefálico y D0,03 cc del lóbulo temporal ipsilateral se mantuvieron dentro de los límites de seguridad, lo que sugiere que el ZAP-X GRS administra la radiación al objetivo con una rápida disminución, minimizando el daño colateral a los tejidos circundantes. El objetivo de futuros estudios será comparar los resultados clínicos y dosimétricos con el uso del colimador de 4 mm. El estudio realizado por Paddick et al. (2023) ofrece una comparación exhaustiva de las plataformas SRS contemporáneas, comparándolas con versiones anteriores de la tecnología. Los resultados revelan avances notables en el rendimiento de los dispositivos actuales, destacando las mejoras en la calidad de los planes de tratamiento. En general, los resultados de este estudio enfatizan que la plataforma ZAP-X es una opción valiosa en la SRS, especialmente para casos que requieren un gradiente de dosis pronunciado y una alta precisión en la localización de lesiones cercanas a estructuras críticas [6,15].
En cuanto a la seguridad, la tasa de complicaciones fue baja, con solo el 16,6 % de los pacientes que experimentaron hipoestesia facial ipsilateral leve. Este efecto secundario es coherente con estudios previos sobre la SRS para la NT y suele ser transitorio. Solo un pequeño porcentaje de pacientes experimentó recurrencia del dolor, lo que se encuentra dentro del rango esperado para la SRS. El uso de la GRS con ZAP-X como tratamiento de segunda línea tras la recurrencia parece ser una opción viable, ya que muestra resultados positivos tras el retratamiento [1,4,10-14].
Conclusiones
En general, los resultados de este estudio sugieren que la ZAP-X GRS es una nueva tecnología prometedora para el tratamiento de la NT refractaria, ya que proporciona altas tasas de alivio del dolor, complicaciones mínimas y una administración precisa de la dosis en el nervio trigémino. Dados los resultados favorables observados en esta cohorte, se necesitan más estudios con poblaciones de pacientes más amplias y seguimientos más prolongados para evaluar plenamente la eficacia y la seguridad a largo plazo de este enfoque. Sin embargo, estos primeros resultados son alentadores y respaldan el uso de ZAP-X como una opción de tratamiento segura, eficaz y mínimamente invasiva para los pacientes con neuralgia del trigémino refractaria.
Referencias
- Tuleasca C, Régis J, Sahgal A, et al.: Stereotactic radiosurgery for trigeminal neuralgia: a systematic review. J Neurosurg. 2019, 130:733-57. 10.3171/2017.9.JNS17545
- Lovo EE, Moreira A, Barahona KC, Caceros V, Cruz C, Arias J: Radioneuromodulation by dual-target irradiation in pain crisis from trigeminal neuralgia. Cureus. 2022, 14:e20971. 10.7759/cureus.20971
- Régis J: Radiosurgery as neuromodulation therapy!. Acta Neurochir Suppl. 2013, 116:121-6. 10.1007/978-3-7091-1376-9_19
- Schneider MB, Walcott B, Adler JR Jr: Neuromodulation via focal radiation: radiomodulation update. Cureus. 2021, 13:e14700. 10.7759/cureus.14700
- Romanelli P, Chuang C, Meola A, Bodduluri RM, Adler JR Jr: ZAP-X: A novel radiosurgical device for the treatment of trigeminal neuralgia. Cureus. 2020, 12:e8324. 10.7759/cureus.8324
- Weidlich GA, Chung W, Kolli S, Thirunarayanan I, Loysel T: Characterization of the ZAP-X® peripheral dose fall-off. Cureus. 2021, 13:e13972. 10.7759/cureus.13972
- Hendricks BK, DiDomenico JD, Barani IJ, Barranco FD: ZAP-X gyroscopic radiosurgery system: a preliminary analysis of clinical applications within a retrospective case series. Stereotact Funct Neurosurg. 2022, 100:99-107. 10.1159/000519862
- Srivastava SP, Jani SS, Pinnaduwage DS, et al.: Treatment planning system and beam data validation for the ZAP-X: A novel self-shielded stereotactic radiosurgery system. Med Phys. 2021, 48:2494-510. 10.1002/mp.14740
- Zhang M, Lamsam LA, Schoen MK, et al.: Brainstem dose constraints in nonisometric radiosurgical treatment planning of trigeminal neuralgia: a single-institution experience. World Neurosurg. 2018, 113:e399-407. 10.1016/j.wneu.2018.02.042
- Timmerman R: A Story of Hypofractionation and the Table on the Wall. Int J Radiat Oncol Biol Phys. 2022, 112:4-21. 10.1016/j.ijrobp.2021.09.027
- Romanelli P, Conti A, Bianchi L, Bergantin A, Martinotti A, Beltramo G: Image-guided robotic radiosurgery for trigeminal neuralgia. Neurosurgery. 2018, 83:1023-30. 10.1093/neuros/nyx571
- Romanelli P, Conti A, Redaelli I, Martinotti AS, Bergantin A, Bianchi LC, Beltramo G: Cyberknife radiosurgery for trigeminal neuralgia. Cureus. 2019, 11:e6014. 10.7759/cureus.6014
- Lee S, Lee JI: Gamma knife radiosurgery for trigeminal neuralgia: review and update. J Korean Neurosurg Soc. 2022, 65:633-9. 10.3340/jkns.2021.0303
- Kerolus MG, Sen N, Mayekar S, et al.: TrueBeam radiosurgery for the treatment of trigeminal neuralgia: preliminary results at a single institution. Cureus. 2017, 9:e1362. 10.7759/cureus.1362
- Paddick I, Mott J, Bedford J, et al.: Benchmarking tests of contemporary SRS platforms: have technological developments resulted in improved treatment plan quality?. Pract Radiat Oncol. 2023, 13:e451-9. 10.1016/j.prro.2023.05.005
Artículo publicado en: https://www.cureus.com/articles/350460-gyroscopic-radiosurgery-for-the-treatment-of-trigeminal-neuralgia#!/